The Center for Information and Study on Clinical Research Participation (CISCRP) is a first-of-its-kind nonprofit organization dedicated to educating and informing the public, patients, medical/research communities, the media, and policy makers about clinical research and the role each party plays in the process.
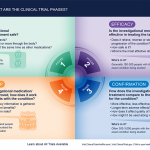
Understanding Clinical Trials
Centerwatch. The global source for clinical trials information: offering news, analysis, study grants, career opportunities, and trial listings to professionals and patients.
Clinical Trials.gov is a NIH resource which provides information on clinical trials such as status of trials and locations of study site across the United States.